Elias James Corey Ph.D.
The Nobel Prize in Chemistry 1990
Organic Chemist - Interested in application of organic chemistry to human health. His groups have achieved a multitude of total syntheses of complex molecules. Originator of retrosynthetic analysis. Recognized as "Most Cited Author in Chemistry" by American Chemical Society in 2002.
"Organic chemistry was especially fascinating with its intrinsic beauty and its great relevance to human health."
Patents
| Publication: | 1/19 |
| Publication No: | USRE41614E1 |
| Title: | Synthetic analogs of ecteinascidin-743 |
| Publication Type: | Grant |
| Publication Date: | Aug 31, 2010 |
| Filing Date: | Dec 17, 2003 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College |
| Abstract: | No Abstract |
| Representative Figure: | No Figure |
| Family | |
| Details | Google Patents USPTO Patent Database |
| Publication: | 2/19 |
| Publication No: | US 7511156 B2 |
| Title: | Analogs of Salinosporamide A |
| Publication Type: | Grant |
| Publication Date: | Mar 31, 2009 |
| Filing Date: | Oct 9, 2006 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College |
| Abstract: | Disclosed herein are analogs of Salinosporamide A, having the Formulae Ia-IVa as follows: |
| Representative Figure: | 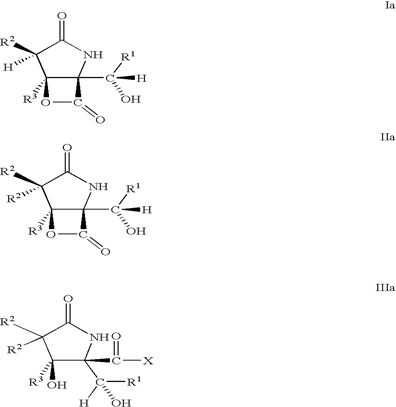 |
| Family | |
| Details | Google Patents USPTO Patent Database |
| Publication: | 3/19 |
| Publication No: | US 20070060561 A1 |
| Title: | Proteasome inhibiting .beta.-lactam compounds |
| Publication Type: | Application |
| Publication Date: | Mar 15, 2007 |
| Filing Date: | Sep 12, 2005 |
| Inventors: | Elias J. Corey |
| Original Assignee: | Corey Elias J, Hogan Philip C |
| Abstract: | No Abstract |
| Representative Figure: | No Figure |
| Family | |
| Details | Google Patents USPTO Patent Database |
| Publication: | 4/19 |
| Publication No: | US 20050228186 A1 |
| Title: | Simple stereocontrolled synthesis of salinosporamide A |
| Publication Type: | Application |
| Publication Date: | Oct 13, 2005 |
| Filing Date: | Apr 9, 2004 |
| Inventors: | Elias J. Corey |
| Original Assignee: | Elias J. Corey |
| Abstract: | No Abstract |
| Representative Figure: | No Figure |
| Family | |
| Details | Google Patents USPTO Patent Database |
| Publication: | 5/19 |
| Publication No: | PCTUS0104376 |
| Title: | Synthetic process for an intermediate for ecteinascidin and phthalascidin compounds |
| Publication Type: | Application |
| Publication Date: | Nov 9, 2004 |
| Filing Date: | Aug 7, 2002 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College |
| Abstract: | No Abstract |
| Representative Figure: | No Figure |
| Family | |
| Details | Google Patents USPTO Patent Database |
| Publication: | 6/19 |
| Publication No: | 6,576,781 |
| Title: | Synthesis of pseudopterosin compounds |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | June 27, 2001 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | The present invention is directed to the synthetic processes outlined in Schemes 1, 2 and 3, to the novel intermediates recited therein, and to the uses of these compounds as synthetic precursors to the pseudopterosins. Other embodiments and aspects of the present invention include the novel synthetic procedures described herein. Scheme I is shown below: ##STR1## |
| Representative Figure: | No Figure |
| Family | 24863947 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 7/19 |
| Publication No: | 6,569,859 |
| Title: | Synthetic analogs of ecteinascidin-743 |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | February 14, 2002 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | The present invention is directed to the synthesis and characterization of compounds having the formula: ##STR1## wherein R1, R2, R3, R4, R5, R6, R7, R8 and R9 are each independently selected from the group consisting of H, OH, OR', SH, SR', SOR', SO2 R', NO2, NH2, NHR', N(R')2, NHC(O)R', CN, halogen, .dbd.O, C(.dbd.O)H, C(.dbd.O)R', CO2 H, CO |
| Representative Figure: | No Figure |
| Family | 24030242 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 8/19 |
| Publication No: | 6,423,877 |
| Title: | Borane-sulfide hydroboration agents |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | November 15, 2000 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | The present invention is directed to a new synthetic route to pseudopterosin aglycone (3): ##STR1## a key intermediate for the synthesis of a group of antiinflammatory natural products including pseudopterosin A (1) and E (2). The pathway of synthesis starts with the abundant and inexpensive (S)-(-)-limonene and its long-known cyclic hydroboration product (4) and leads to the chiral hydroxy ketone (6). Conversion of (6) to (10) followed by a novel aromatic annulation produced (15) which underwent highly diastereoselective cyclization to afford the protected pseudopterosin aglycone (16). The naturally occurring pseudopterosins such as (1) and (2) are readily available from this key intermediate. ##STR2## |
| Representative Figure: | No Figure |
| Family | 26861527 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 9/19 |
| Publication No: | 6,348,467 |
| Title: | Synthetic analogs of ecteinascidin-743 |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | February 22, 2000 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | The present invention is directed to the synthesis and characterization of compounds having the formula: ##STR1## wherein R1, R2, R3, R4, R5, R6, R7, R8 and R9 are each independently selected from the group consisting of H, OH, OR', SH, SR', SOR', SO2 R', NO2, NH2, NHR', N(R')2, NHC(O)R', CN, halogen, .dbd.O, C(.dbd.O)H, C(.dbd.O)R', CO2 H, CO2 R', C1 -C12 alkyl, C2 -C12 alkenyl, C2 -C12 alkynyl, substituted or unsubstituted aryl, substituted or unsubstituted aralkyl, and substituted or unsubstituted heteroaromatic; wherein each of the R' groups is independently selected from the group consisting of H, OH, NH2, NO2, SH, CN, halogen, .dbd.O, C(.dbd.O)H, C(.dbd.O)CH3, CO2 H, CO2 CH3, C1 -C12 alkyl, C2 -C12 alkenyl, C2 -C12 alkynyl, aryl, aralkyl, and heteroaromatic; wherein each dotted circle represents one, two or three optional double bonds; wherein R7 and R8 may be joined into a carbocyclic or heterocyclic ring system; and wherein X.sub.1 and X.sub.2 are each independently defined as above for R1 -R8, and each further includes specific preferred groups as defined herein. |
| Representative Figure: | No Figure |
| Family | 22600906 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 10/19 |
| Publication No: | 6,348,467 |
| Title: | Synthetic analogs of ecteinascidin-743 |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | February 22, 2000 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | The present invention is directed to the synthesis and characterization of compounds having the formula: ##STR1## wherein R1, R2, R3, R4, R5, R6, R7, R8 and R9 are each independently selected from the group consisting of H, OH, OR', SH, SR', SOR', SO2 R', NO2, NH2, NHR', N(R')2, NHC(O)R', CN, halogen, .dbd.O, C(.dbd.O)H, C(.dbd.O)R', CO2 H, CO2 R', C1 -C12 alkyl, C2 -C12 alkenyl, C2 -C12 alkynyl, substituted or unsubstituted aryl, substituted or unsubstituted aralkyl, and substituted or unsubstituted heteroaromatic; wherein each of the R' groups is independently selected from the group consisting of H, OH, NH2, NO2, SH, CN, halogen, .dbd.O, C(.dbd.O)H, C(.dbd.O)CH3, CO2 H, CO2 CH3, C1 -C12 alkyl, C2 -C12 alkenyl, C2 -C12 alkynyl, aryl, aralkyl, and heteroaromatic; wherein each dotted circle represents one, two or three optional double bonds; wherein R7 and R8 may be joined into a carbocyclic or heterocyclic ring system; and wherein X.sub.1 and X.sub.2 are each independently defined as above for R1 -R8, and each further includes specific preferred groups as defined herein. |
| Representative Figure: | No Figure |
| Family | 22600906 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 11/19 |
| Publication No: | 5,721,362 |
| Title: | Process for producing ecteinascidin compounds |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | September 18, 1996 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | The present invention is directed to a synthetic process for the formation of ecteinascidin compounds and related structures, such as the saframycins. In one particularly preferred embodiment, the present invention provides a synthetic route for the formation of ecteinascidin 743 (1), ##STR1## an exceedingly potent and rare marine-derived antitumor agent which is slated for clinical trials. The process of this invention is enantio- and stereocontrolled, convergent and short. Also disclosed are novel process intermediates, useful not only in the total synthesis of ecteinascidin 743, but also other known ecteinascidin compounds, including derivatives and analogs thereof.n. |
| Representative Figure: | No Figure |
| Family | 24874473 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 12/19 |
| Publication No: | 5,096,892 |
| Title: | Arylsulfatase inhibition and potentiation of angiostatic steroids and heparin |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | May 27, 1988 |
| Inventors: | Folkman; Moses J. (Brookline, MA), Chen; Neil T. (Newton Center, MA), Corey; Elias J. (Cambridge, MA) |
| Original Assignee: | The Children's Medical Center Corporation (Boston, MA) and President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | Anigiogenesis is controlled by administering to a mammal an effective amount of an inhibitor of arylsulfatase. Preferably, the arylsulfatase inhibitor is administered in a pharmaceutically acceptable vehicle in combination with an angiostatic steroid and (optionally) heparin (by which term we include all forms and fragments of heparin having the desired angiostatic activity). Hydrocortisone is one specifically preferred steroid. The preferred arylsulfatase inhibitor is a carboxylic acid ester or a sulfuric acid ester of a benzylic alcohol, most preferably the esters defined more particularly below. The arylsulfatase inhibitor is preferably administered locally to the tissue experiencing undesired angiogenesis. Arylsulfatase inhibitor and an angiostatic steroid are included in a pharmaceutically acceptable vehicle, preferably also with heparin, to yield an angiostatic thereapeutic composition. Also, coagulation of blood is inhibited by adding a composition comprising an arylsulfatase inhibitor and heparin to the blood. Such compositions are also disclosed. |
| Representative Figure: | No Figure |
| Family | 24874473 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 13/19 |
| Publication No: | 4,943,635 |
| Title: | Enantioselective reduction of ketones |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | July 29, 1988 |
| Inventors: | Elias J. Corey |
| Original Assignee: | President and Fellows of Harvard College (Cambridge, MA) |
| Abstract: | Chiral 1,3,2-oxazaborolidines and tetrahydro-1,3,2-oxazaborines are effective catalysts for the borane reduction of prochiral ketones to optically active alcohols. |
| Representative Figure: | No Figure |
| Family | 26782007 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 14/19 |
| Publication No: | 4,214,099 |
| Title: | Intermediates for synthesis of precursors for prostaglandins |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | June 22, 1978 |
| Inventors: | Corey; Elias J. (Cambridge, MA), Bindra; Jasjit S. (Groton, CT), Schaaf; Thomas K. (Old Lyme, CT) |
| Original Assignee: | Pfizer Inc. (New York, NY) |
| Abstract: | A new synthesis of key prostaglandin precursors and intermediates employed in their preparation. The novel synthetic sequence of this invention is shorter and more efficient than those previously employed to prepare to key intermediate. |
| Representative Figure: | No Figure |
| Family | |
| Details | Google Patents USPTO Patent Database |
| Publication: | 15/19 |
| Publication No: | 4,122,093 |
| Title: | Process for preparing a lactone |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | April 14, 1977 |
| Inventors: | Corey; Elias J. (Cambridge, MA), Bindra; Jasjit S. (Groton, CT), Schaaf; Thomas K. (Old Lyme, CT) |
| Original Assignee: | Pfizer Inc. (New York, NY) |
| Abstract: | A new synthesis of key prostaglandin precursors and intermediates employed in their preparation. The novel synthetic sequence of this invention is shorter and more efficient than those previously employed to prepare to key intermediate. |
| Representative Figure: | No Figure |
| Family | 27410789 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 16/19 |
| Publication No: | 3,992,439 |
| Title: | Synthesis of prostaglandins of the "one"-series |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | February 14, 1975 |
| Inventors: | Schaaf; Thomas K. (Old Lyme, CT), Corey; Elias J. (Cambridge, MA) |
| Original Assignee: | Pfizer Inc. (New York, NY) |
| Abstract: | In the synthesis of prostaglandins of the "one" -series an alteration in the conventional reaction sequence avoids side reactions and provides an improved synthesis via a series of novel intermediates. In this improved synthesis the side chain at the 8 position is attached to the five membered ring before the side chain at the 12 position is attached. |
| Representative Figure: | No Figure |
| Family | 26936861 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 17/19 |
| Publication No: | 3,992,438 |
| Title: | Novel prostaglandin intermediates |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | November 19, 1975 |
| Inventors: | Corey; Elias J. (Cambridge, MA), Bindra; Jasjit S. (Groton, CT), Schaaf; Thomas K. (Old Lyme, CT) |
| Original Assignee: | Pfizer Inc. (New York, NY) |
| Abstract: | A new synthesis of key prostaglandin precursors and intermediates employed in their preparation. The novel synthetic sequence of this invention is shorter and more efficient than those previously employed to prepare to key intermediate. |
| Representative Figure: | No Figure |
| Family | |
| Details | Google Patents USPTO Patent Database |
| Publication: | 18/19 |
| Publication No: | 3,974,183 |
| Title: | Reduction of trans-1-(2-carboxymethyl-3-hydroxy-5-p-phenylbenzoyloxycyclopentyl)-1-oc ten-3-one-.gamma.-lactone |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | July 12, 1974 |
| Inventors: | Elias J. Corey |
| Original Assignee: | Pfizer Inc. (New York, NY) |
| Abstract: | A process for reducing trans-1-(2-carboxymethyl-3-hydroxy-5-acyloxycyclopentyl)-1-octen-3-one-.ga mma.-lactone to the corresponding 3-ol which makes use of trihydrocarbylborohydride reagents, some of which are new, e.g. lithium 2-thexyl-8-methyl-2-borabicyclo-[3,3,1]nonylhydride. A new process for producing this and other lithium thexyl-dihydrocarbylborohydrides which comprises contacting a thexyl-dihydrocarbylborane with an organo-lithium compound having a .beta.-hydrogen atom. |
| Representative Figure: | No Figure |
| Family | 26984501 |
| Details | Google Patents USPTO Patent Database |
| Publication: | 19/19 |
| Publication No: | 3,943,151 |
| Title: | Prostaglandin intermediates |
| Publication Type: | |
| Publication Date: | |
| Filing Date: | October 24, 1973 |
| Inventors: | Corey; Elias J. (Cambridge, MA), Bindra; Jasjit S. (Groton, CT), Schaaf; Thomas K. (Old Lyme, CT) |
| Original Assignee: | Pfizer Inc. (New York, NY) |
| Abstract: | A new synthesis of key prostaglandin precursors and intermediates employed in their preparation. The novel synthetic sequence of this invention is shorter and more efficient than those previously employed to prepare to key intermediate. |
| Representative Figure: | No Figure |
| Family | 23618926 |
| Details | Google Patents USPTO Patent Database |
Discover Your Abilities and Aspirations!
 $10 $25 $50 $100 Other
$10 $25 $50 $100 Other
Tax Exempt 501(c)3 Non-Profit Organization
Any Currency
“…the peace that is found in libraries and laboratories…” - Louis Pasteur
Copyright © 2023 Ganga Library Inc. All Rights reserved.;

Photo: Elias .J. Corey at Harvard. Creator: Trvthchem personal photo. 19 Nov 2007. Source: Wikimedia Commons.
Name: Elias James Corey
Birth: 12 July 1928, Methuen, MA, USA
Institution: Harvard University, Cambridge, MA, USA
Award: "for his development of the theory and methodology of organic synthesis"
Subject: organic chemistry
National Medal of Science Chemistry 1988
Biography
Nobel Lecture
Publications
Books
Patents
Thesis
History of Discovery
Honoring Corey
Images
Videos













